Clear solutions infusion filters
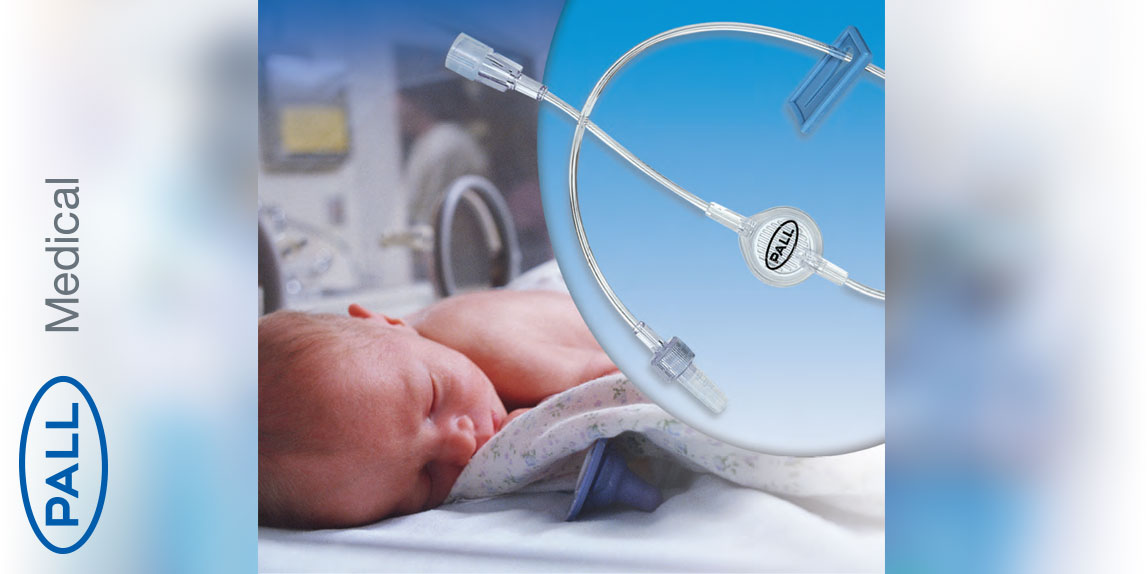
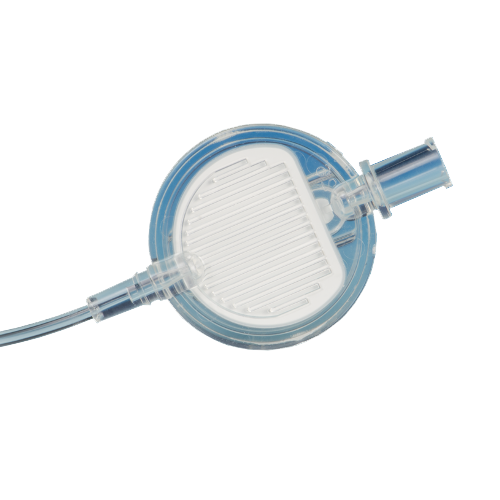
Clear solution infusion filters - adult / neonatal
Particulate contamination occurs from a variety of sources, intrinsic in infusion devices and equipment or an external cause is that represented by the manner of handling. Particles cause phlebitis in peripheral infusion lines and have serious systemic effects, damaging the lungs and solid organs by irritation of the endothelium and deposition in the microvasculature.
Clear solution infusion filters - pediatric / cytostatic administration
Microbial contamination - of IV administration systems occurring accidentally through handling. Some bacteria proliferate rapidly in infusion fluids, increasing the risk of infection. Entrained air - results from outgassing of solutions, incomplete priming or when connections are loosened. Entrained air is especially problematic on central lines, leading to gas embolisms.
Lipid solutions infusion filters
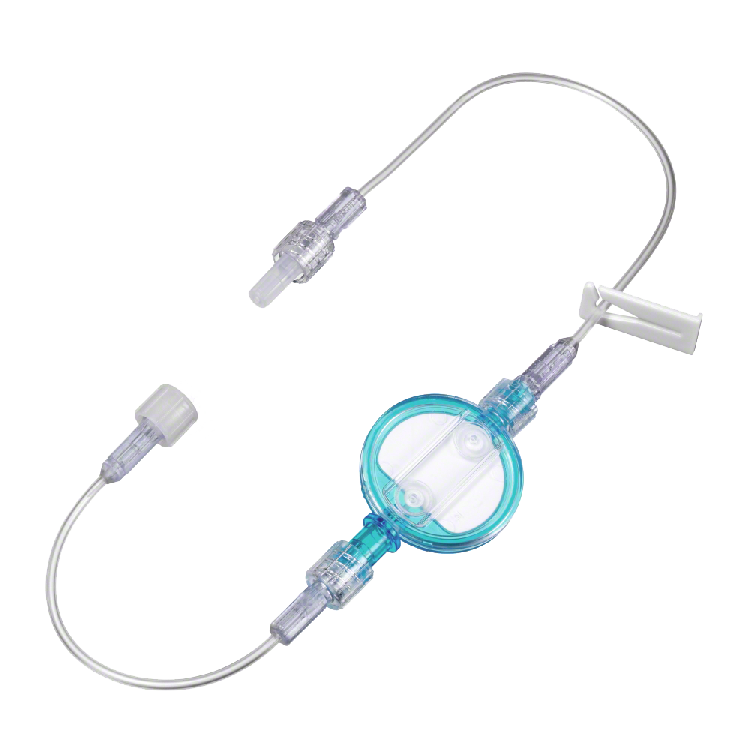
Lipid solution infusions filters - adults
The filter set is an air removal filter for use with any IV nutritional administration containing lipids and lipid emulsions. It is indicated for the removal of particulate residues, microbial contaminants and entrained air that may be found in solutions used in IV administration. It provides the patient with protection against contamination from particulate, oversized lipid droplets, microorganisms, and air.
Lipid solution infusion filters - adult / neonatal / pediatric
Accidental contamination of intravenous solutions can have serious consequences for patients. Particulate contamination, shown to cause phlebitis, may be inherently present in the infusion preparation. It may also arise from contact of the solution with equipment or from handling of the solution. Entrained air, resulting from solution degassing, incomplete priming or detachment of the infusion line, has been shown to be particularly problematic on central lines, leading to gas emboli. Oversized lipid droplets occur in mixtures due to instability.
Intubation / respiratory filters
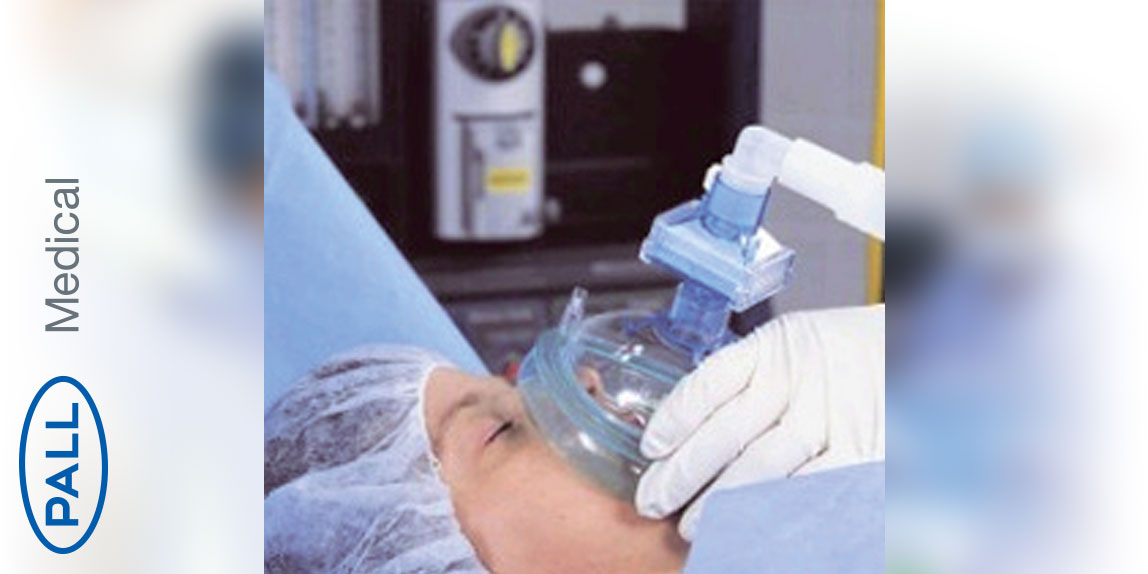

Pediatric intubation/anesthesia filters with monitoring port
High efficiency bacterial/viral filter with heat and moisture exchanger for anaesthesia. The filter is indicated for the prevention of cross infection through breathing systems in anaesthesia. The filter has also been validated to retain prion proteins and allergens. Routine use of this device will protect the interior of the breathing system, allowing for extended use of the breathing system in conjunction with protocol that ensures hygiene and mechanical integrity of the system.
Adult intubation filters with monitoring port
The breathing filter is a high efficiency bacterial/viral filter with heat and moisture exchanger for patient end use. The filter is indicated for the prevention of cross infection through breathing systems in anaesthesia and for the reduction of associated pneumonia in long-term ventilation.
Laparoscopic smoke filters
The laparoscopic smoke filter allows safe and quick evacuation of surgical smoke throughout the surgical procedure. It reduces patient and staff exposure to the harmful biological and chemical components of surgical smoke.
Sterile water filters without autoclaving


Sterile water filters
The use of the interchangeable capsule filter provides water with a sterilisation degree of 0.2 µm at the time of use. The integrated high-tech pre-filtration layer provides exceptional particle trapping capacity with good flow rates and protects the double-sterilization grade membranes allowing long life and high performance. The filter capsule retains fungi, protozoa and bacteria such as Legionella, Pseudomonas, non-tuberculous mycobacteria and Escherichia coli without volume restrictions.
Sterile water filters for shower
The water filter provides filtered water for medical use, personal hygiene, wound care, consumption and preparation of cold beverages, food and for rinsing medical instruments. The double sterilisation grade membrane is rated and validated to 0.2 µm and protects against particles and pathogens in water such as Legionella and Pseudomonas.
Protective equipment


• Protective equipment cat. III-a for chemotherapy and clean rooms
• FFP3 and FFP2 respiratory masks
• Cytostatic protection gloves
• Medical devices for oncology
• Closed cytostatic transfer and delivery systems